Electrochemical Anodization, Porous Anodic Alumina
Porous Anodic Alumina (PAA) is formed by the anodization of Al in appropriate acidic or basic electrolytic solutions. The recent improvement in the degree of ordering of the anodic porous alumina has increased the attractiveness of this material from the viewpoint of nanofabrication. It is used as a starting template for the fabrication of several kinds of nanodevices due to its fine porous structure with high aspect ratio. The geometrical structure of anodic porous alumina is schematically represented as a honeycomb structure consisting of a close-packed array of columnar alumina units called cells, each containing a central straight hole (see figure below).
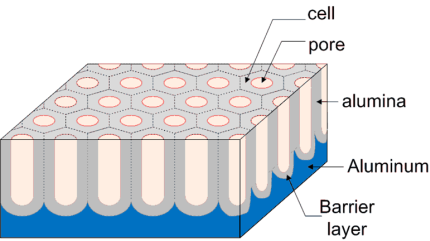
The dimensions of the anodic porous alumina cells depend on the anodizing conditions. The cell size, which is equivalent to the hole interval, is determined by the applied voltage used for the anodization; the cell size has a good linear relationship with the applied voltage. The value of the constant of the cell size divided by the applied voltage is approximately 2.5 nm/V. The hole size is dependent on the electrolyte composition, temperature, period of anodization as well as applied voltage. The hole size is also controlled by the porewidening treatment by dipping the porous alumina in an appropriate acid solution after the anodization. The cell size usually ranges from 10 to 500 nm and the hole size from 5 to 400 nm depending on the anodizing and post-anodizing conditions. The depth of the holes (thickness of the oxide films) has a good linear relationship with the period of the anodization.

1. Cooling Stage
2. Teflon Cell
3. Aluminum Sample (Anode)
4. Platinum Gauge (Cathode)
5. Stirring Mechanism
6. Electrolytic Solution
The above figure shows an apparatus of our electrochemical experiment. The electrochemical cell consists of a two-electrode system, e.g., the Platinum (Pt) mesh acting as the counter electrode (4) and Al sheet acting as the working electrode (3). The Al sheet is inserted between the electrolyte container (2) and a Peltier cooling plate (1) fixed by screws. To stir the electrolytes vigorously, a motor-controlled agitator is used (5). The apparatus is operated by a computer, using home-made software. The temperature of the electrolyte is held at the desired set point by a cooling apparatus, a Peltier element driven by a PID controller.
Photo of the experimental set up.

SEM image of a Porous Anodic Alumina produced by our experimental set up.